Unless some Tony Stark comes to save the day with his marvelous invention of Arc Reactor, we are still distant from a future of unlimited power supply. For the majority of the time, we are mostly dependent on batteries for providing juice to our favorite portable devices. All the consumer electronics uses one of the two types of rechargeable battery: Lithium-ion or Lithium Polymer.
The main popularity behind Lithium-ion batteries is due to the fact that it is light-weighted, and has high energy density. They are extensively used in mobile phones, cameras, cars, etc. In this article, we are going to discuss everything you need to know about Lithium-ion batteries including their working principle, advantages, and some of the hidden perks as well.
Also Read: What is Artificial Intelligence (AI)?
What is a Lithium Ion Battery?
As the name suggests, lithium-ion batteries use lithium ions to generate power required by our devices. It is a very light metal with high energy density. What is energy density? Well, energy density is the amount of energy that is stored per unit volume of the battery. Furthermore, this property enables the battery to be light.
Though lithium has a lot of advantages we cannot use it to power up portable electronics due to its metallic nature. So, shifting to lithium-ions is the only solution. Moreover, they have the same properties. The only difference between the two is that one is metallic and the other one is non-metallic in nature which makes it ideal to use for different purposes.
History of Lithium-Ion Battery
The term Lithium-Ion battery was first coined back in 1912, by G.N Lewis. But, it was somewhere around the 1970s when the first non-rechargeable Lithium-ion battery was introduced commercially to the market. The ’80s were the time, with several unsuccessful attempts were made to make Lithium-ion batteries rechargeable. Only in 1991, the world’s first rechargeable Lithium-ion battery was invented which we see in our day-to-day life.
Working Principle of Lithium-Ion Battery
We all know that batteries are made up of cells and each cell consists of three parts: anode (positive electrode), cathode (negative electrode), and a liquid electrolyte. The anode of the lithium-ion battery is crafted using carbon and the cathode is made of Cobalt oxide or any other metal oxide. A simple salt solution (liquid electrolyte) containing lithium-ion would be used connecting these two electrodes.
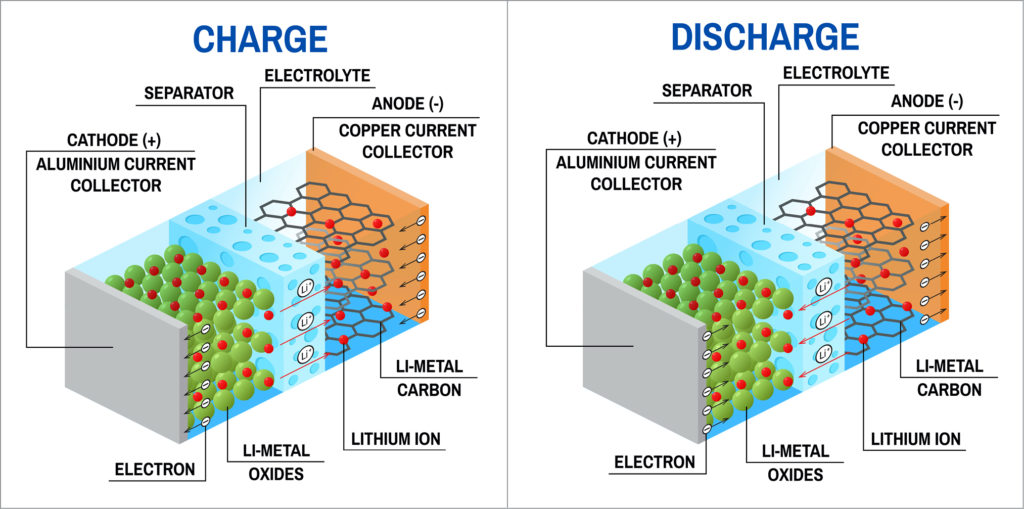
When the lithium-ion batteries in your laptop or any other device are powering them then the positively charged lithium ions move towards the positive electrode via the electrolyte. Whereas, the electrons move from the positive to the negative electrode. The exact opposite thing happens, while you’re charging the lithium-ion batteries. As long as the cycle keeps on going the Lithium-ion batteries would power up your device.
Introduction to Lithium-ion Battery
Now that we are done with the working principle of the Li-ion battery, it’s the best time to jump right into its practical usage. For an easier understanding of our readers, we would keep the section as lucid as possible (trying hard not to geek out!).
Nominal Voltage: The actual voltage rating of the Li-ion battery is termed as nominal voltage. Regardless of the manufacturer, the nominal voltage is always set to 3.6V by default.
Full Discharge Voltage: By any means necessary, the voltage of any Lithium-ion battery shouldn’t drop beyond 3.2V. Doing so comes with its own sets of disadvantages. It can either alter the internal resistance of the battery or damage it permanently.
mAh Rating: The mAh stands for Milli Ampere hour. This is the standard used for rating the capacity of your Li-ion battery. So, the mAh rating directly correlates to the overall run-time of your battery.
C Rating: The C Rating is another important aspect to look for while getting a Lithium-ion battery. The C Rating denotes the maximum current that can be drawn from the battery.
Care Tips for Lithium-ion Battery
The overall longevity of the battery heavily depends upon its usage. Here are some of the few tips on how you can increase the overall life cycle of your Li-ion battery.
1. Partially Discharge the Battery
You should always recharge your Li-ion battery when it’s at 20% to 30% capacity. This, in turn, can significantly extend the life cycle of your battery. One of the main reasons being, full discharge can bring down the voltage below the standard 3.2V.
2. Avoid Charging it to Full Capacity
The same is applicable for changing your battery to its full capacity. Now, we are not suggesting you do the same all the time. In case you are traveling, or about to store your device for a longer duration of time. You can always charge the battery to its 100% capacity.
3. Keep Track of Battery Temperature
Limiting the temperature for your Lithium-ion battery can extend its time to a certain degree. Higher temperatures would always heat up the battery make it unstable and unsafe to use. The same goes for using it below a certain temperature threshold.
4. Use Proper Charging Methods
Every Lithium-ion battery comes with its own charging rating. In the era of fast charging, going beyond the limit can always damage the cells.
Related: 5 Things You Should Know Before Buying a Power Bank
The Bottom Line
Lithium-ion batteries are used to fuel different consumer electronics including earbuds, laptops, mobiles, and more. Lithium-ion batteries are the popular choice in military and aerospace applications as they tend to have a lower self-discharge rate, higher energy density, voltage capacity when compared to traditional batteries. Furthermore, they can be recharged as many as a hundred times and are stable as well.













